DTaP Vaccine Market Size
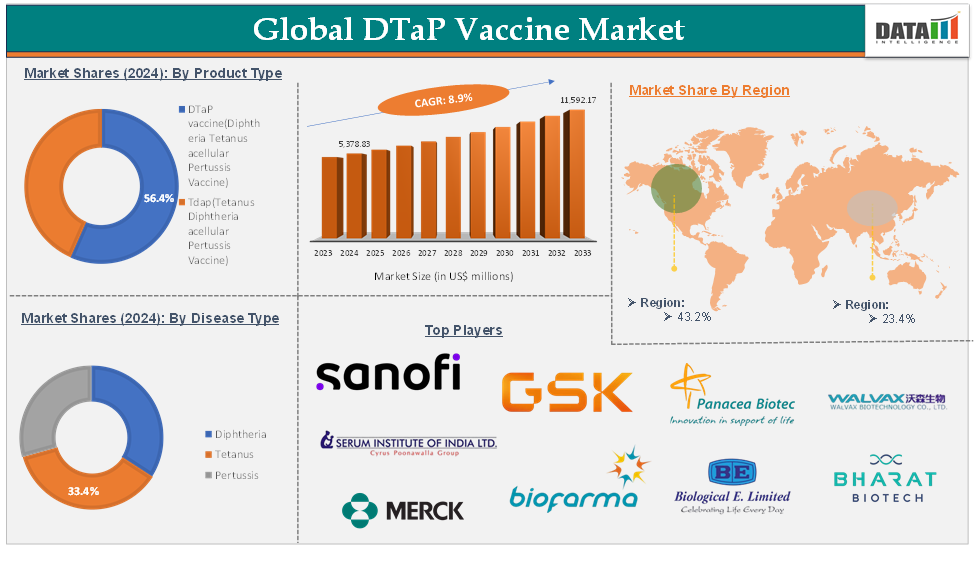
Global DTaP vaccine market reached US$ 5,378.83 Million in 2024 and is expected to reach US$ 11,592.17 Million by 2033, growing at a CAGR of 8.9% during the forecast period of 2025-2033.
DTaP stands for diphtheria, tetanus, and acellular pertussis vaccine combination shot designed to protect children from these three serious bacterial diseases. In the United States, DTaP is routinely given as a five-dose series to infants and young children at 2, 4, and 6 months, 15–18 months, and 4–6 years of age. The vaccine uses inactivated components: diphtheria and tetanus toxoids, and purified antigens from the pertussis bacterium, making it safe and effective for young children.
Market key trends include increasing adoption of combination vaccines, which streamline immunization schedules and improve compliance. Significant investment in R&D, including the use of artificial intelligence for vaccine development and strategic acquisitions.
Key drivers in the market industry include the growing incidence of infectious diseases, especially pertussis outbreaks, which are fueling demand for effective immunization. Rising adoption of combination vaccines, government policies, and universal immunization programs, such as India's universal immunization program, supports widespread vaccine adoption. Advancements in vaccine technology and the introduction of high-valent and combination vaccines.
Expansion into emerging economies like India, China, Brazil, and Indonesia, where increasing healthcare investments and rising populations create significant growth potential, and the development of new combination vaccines and innovative delivery methods to address diverse healthcare needs is a significant opportunity for the market.
DTaP Vaccine Market – Executive Summary
For more details on this report, Request for Sample
Market Scope
| Metrics | Details | |
| CAGR | 8.9% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Mn) | |
| Segments Covered | Product Type | DTaP Vaccine (Diphtheria Tetanus acellular Pertussis Vaccine), Tdap (Tetanus Diphtheria acellular Pertussis Vaccine) |
| Disease Type | Diphtheria, Tetanus, Pertussis | |
| Age Group | Pediatric, Adult | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
DTaP Vaccine Market Dynamics: Drivers & Restraints
Rising adoption of combination vaccines
The growing preference for combination vaccines is a key driver in the global DTaP vaccine market. By streamlining immunization schedules and reducing the number of clinic visits, combination vaccines help increase vaccination coverage rates, reduce healthcare costs, and improve overall public health outcomes. As a result, the demand for DTaP-containing combination vaccines is expected to continue rising, fueling growth in the global market.
Common combination vaccines for children-such as Pediarix, Pentacel, Kinrix, Quadracel, Vaxelis, and ProQuad-play a key role in driving the global DTaP vaccine market by simplifying immunization schedules and improving compliance. These vaccines combine protection against multiple diseases (like diphtheria, tetanus, pertussis, polio, hepatitis B, and Haemophilus influenzae type B) into a single shot, reducing the number of injections needed and making it easier for parents and healthcare providers to ensure children are fully immunized.
According to the ScienceDirect Vaccines data in April 2025, Diphtheria-tetanus-pertussis-containing vaccines (DTPCVs) are at the leading edge of combination vaccine innovation worldwide. However, the approval and availability of high-valent DTPCVs differ across countries due to varying regulatory standards. To meet urgent public health demands, some national regulatory authorities (NRAs) have implemented more flexible and expedited pathways to accelerate the licensing and adoption of these essential vaccines.
The convenience and efficiency of these combination vaccines have led to their widespread adoption, fueling market growth as the pediatric population rises and demand for streamlined, comprehensive immunization increases. All these factors demand the global DTaP vaccine market.
Supply chain and distribution challenges
The supply chain and distribution challenges will hinder the growth of the global DTaP vaccine market. DTaP vaccines are biologics that require complex manufacturing processes and cold chain logistics. Disruptions in production (e.g., due to facility upgrades or regulatory issues) or transportation delays can result in shortages or uneven distribution, limiting access in some regions.
DTaP vaccines are sensitive biological products that require strict temperature control (typically 2–8°C) during storage and transportation. Any break in the cold chain can compromise vaccine potency, leading to wastage and reduced effectiveness. Maintaining this cold chain is particularly challenging in low-resource settings, rural areas, and regions with unreliable power supplies.
The distribution of vaccines involves multiple steps, including manufacturing, warehousing, transportation, and delivery to healthcare facilities. Each step introduces risks of delays, mishandling, or misplacement. Inadequate infrastructure, limited transportation options, and customs clearance delays can further complicate timely vaccine delivery, especially in remote or conflict-affected regions.
Different countries have varying regulatory requirements for vaccine approval, importation, and distribution. Navigating these regulations can slow down the supply chain, especially when rapid deployment is needed during public health emergencies. Thus, the above factors could be limiting the global DTaP vaccine market's potential growth.
DTaP Vaccine Market - Segment Analysis
The global DTaP vaccine market is segmented based on product type, disease type, age group, and region.
Product Type:
The DTaP vaccine(diphtheria, tetanus, acellular pertussis vaccine) segment was valued at US$ 2,930.97 million in 2024 and is estimated to reach US$ 5,318.69 million by 2033, growing at a CAGR of 6-7% during the forecast period from 2025-2033
DTaP stands for diphtheria, tetanus, and acellular pertussis. The “acellular” part means that the pertussis component uses purified parts of the bacteria, making the vaccine safer and causing fewer side effects compared to older versions.
The DTaP vaccine is a combination immunization that protects against three serious bacterial infections. Diphtheria is a respiratory disease caused by Corynebacterium diphtheriae, which can lead to breathing problems, heart failure, and death. Tetanus is caused by Clostridium tetani. This infection leads to muscle stiffness and spasms, often referred to as "lockjaw." Pertussis (whooping cough) is caused by Bordetella pertussis, It is a highly contagious respiratory disease known for severe coughing fits.
Additionally, key players in the industry and their product launches, approvals that will drive this segment's growth in the market. For instance, in July 2024, the DTaP vaccine is an FDA-approved, five-dose immunization series recommended for young children in the United States to protect against diphtheria, tetanus, and pertussis (whooping cough). DTaP stands for diphtheria, tetanus, and acellular pertussis, and the vaccine helps prevent these serious childhood diseases before age seven. These factors have solidified the segment's position in the global DTaP vaccine market.
DTaP Vaccine Market – Geographical Analysis
The North America DTaP vaccine market was valued at US$ 2,323.65 million in 2024 and is estimated to reach US$ 5,007.82 million by 2033, growing at a CAGR of 7-8% during the forecast period from 2025-2033
In the U.S. and Canada, DTaP vaccination is mandatory for school entry, which ensures high compliance and consistent demand. National programs like the Vaccines for Children (VFC) in the U.S. provide free vaccines, including DTaP, to eligible populations, boosting market penetration.
Public health authorities like the CDC, Health Canada, and organizations like the AAP (American Academy of Pediatrics) run ongoing awareness programs about vaccine-preventable diseases and the importance of the DTaP series. These campaigns help counter vaccine hesitancy and maintain high vaccination rates.
For instance, in July 2024, Haiti’s Ministry of Public Health and Population recently vaccinated over 230,000 children as part of a targeted campaign aimed at reducing infant mortality from diphtheria. Supported by funding from the Government of Canada, the Pan American Health Organization (PAHO/WHO) provided essential technical and logistical assistance to help implement the campaign successfully.
North America is home to major vaccine manufacturers and R&D centers, such as Sanofi, GSK, Merck, and others, driving innovation and supply reliability. Growing adoption of combination vaccines (e.g., DTaP-HepB-IPV) reduces the number of injections per visit, improving patient compliance and provider convenience. Innovations in vaccine formulation and delivery (e.g., fewer side effects, better stability) support product differentiation and adoption.
For instance, in January 2023, the FDA approved the Adacel (Tdap) vaccine for use during the third trimester of pregnancy to help protect infants younger than two months from pertussis (whooping cough), providing crucial protection before infants are old enough to receive their first scheduled vaccine dose at two months of age. Thus, the above factors are consolidating the region's position as a dominant force in the global DTaP vaccine market.
The Asia-Pacific DTaP vaccine market was valued at US$ 1,258.65 million in 2024 and is estimated to reach US$ 2,712.57 million by 2033, growing at a CAGR of 8-9% during the forecast period from 2025-2033
The region’s substantial paediatric population, especially in countries like China and India, creates strong demand for routine childhood immunizations, making Asia Pacific the largest and fastest-growing DTaP market. National initiatives such as India’s Universal Immunization Program and similar efforts across Southeast Asia and China are expanding vaccine coverage and ensuring regular DTaP administration to infants and children.
Countries like India, China, and Japan have significantly scaled up their national immunization programs, including DTaP or pentavalent vaccines that contain DTaP components. Governments are increasingly including combination vaccines in public procurement to meet WHO immunization targets.
For instance, in December 2023, Panacea Biotec announced the launch of EasyFourPol, the world’s first fully liquid wP-IPV-based pentavalent vaccine, in India. EasyFourPol protects children against five serious diseases: diphtheria, tetanus, pertussis (whooping cough), polio, and invasive infections caused by Haemophilus influenzae type b. This innovative, ready-to-use combination vaccine streamlines the immunization process by eliminating the need for reconstitution, reducing the number of injections required, and supporting greater comfort and compliance for children and parents. Thus, the above factors are consolidating the region's position as a dominant force in the global DTaP vaccine market.
DTaP Vaccine Market – Major Players
The major global players in the DTaP vaccine market include Sanofi, GSK plc, Merck & Co., Inc., Serum Institute of India Pvt. Ltd., Bio Farma, Biological E, Panacea Biotec, Walvax Biotechnology Co., Ltd., Bharat Biotech., MassBiologics, and Mitsubishi Tanabe Pharma Corporation among others.
Key Developments
- In July 2022, DAPTACEL is a vaccine produced by Sanofi Pasteur, Ltd., and is officially named "Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed." It is indicated for active immunization against diphtheria, tetanus, and pertussis in infants and children from 6 weeks up to 6 years old.