Cervical Cancer Treatment Market Size
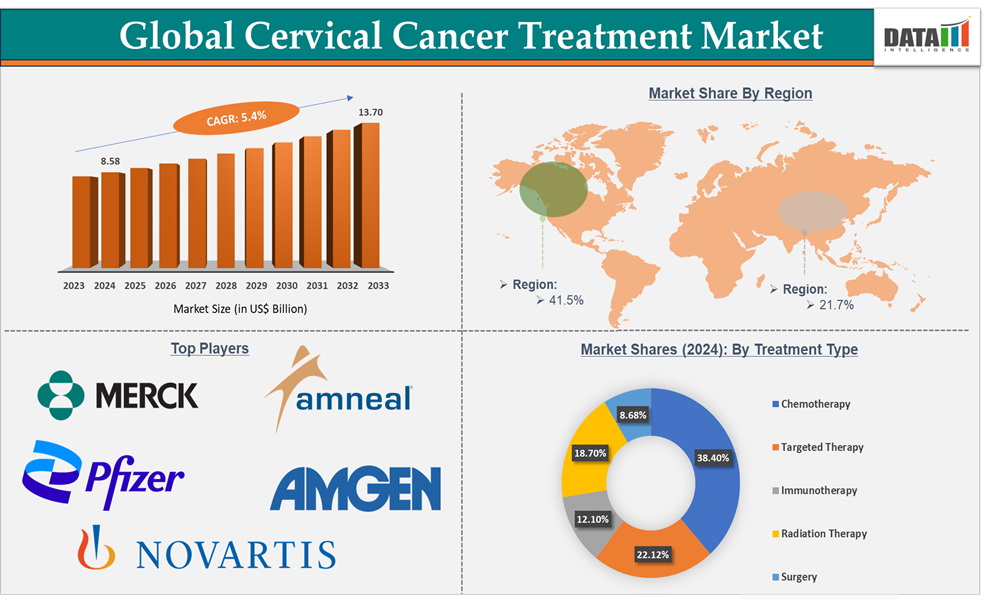
The global cervical cancer treatment market size reached US$ 8.58 billion in 2024 and is expected to reach US$ 13.70 billion by 2033, growing at a CAGR of 5.4% during the forecast period 2025-2033.
Cervical Cancer Treatment Market Overview
The cervical cancer treatment market is a dynamic and rapidly evolving sector driven by increasing global awareness, advancements in medical technologies, and growing emphasis on preventive measures. Cervical cancer, which is primarily caused by persistent HPV (Human Papillomavirus) infection, continues to represent a significant public health challenge worldwide, particularly in low- and middle-income countries (LMICs).
However, early detection, HPV vaccination, and improved treatment options have contributed to a decline in mortality rates in developed regions such as North America, Europe, and the Asia-Pacific. This market is witnessing substantial growth due to the increased adoption of innovative treatment modalities, growing demand for chemotherapy, and rising investments in research and development (R&D).
Cervical Cancer Treatment Market Executive Summary
Cervical Cancer Treatment Market Dynamics: Drivers & Restraints
The rising incidence of cervical cancer is significantly driving the market growth
The rising incidence of cervical cancer is significantly driving the growth of the cervical cancer treatment market in several key ways. As the number of cervical cancer cases increases globally, particularly in regions where screening and vaccination programs are less widespread, there is a corresponding rise in demand for effective treatments.
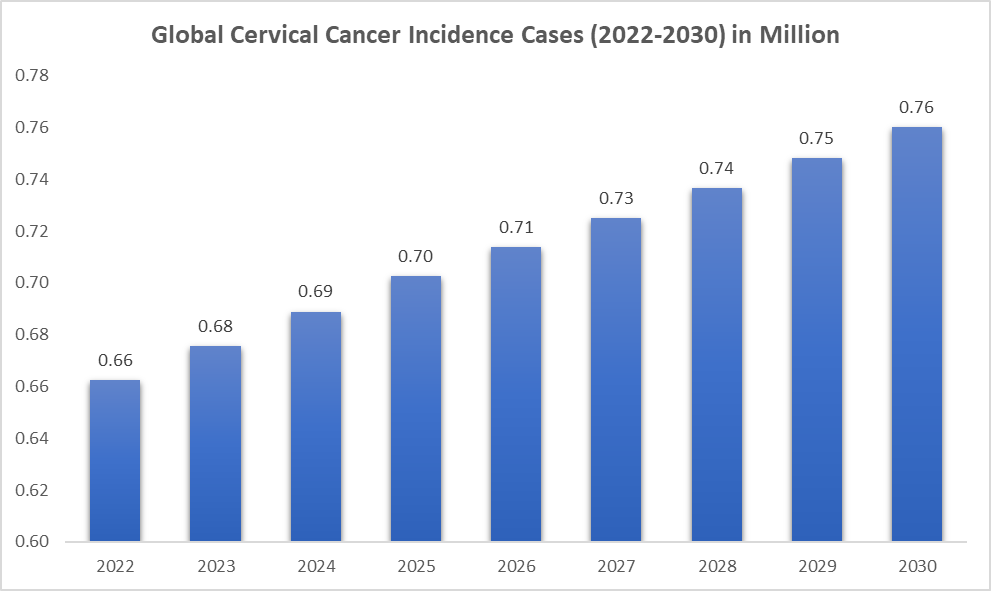
For instance, the World Health Organization (WHO) projected that there is a growing incidence of cervical cancer, with expected incidence cases of 0.70 million in 2025. As more women are diagnosed with cervical cancer due to increased awareness, improved screening programs, and better diagnostic tools, there is an uptick in the demand for medical treatments. This includes surgery, chemotherapy, radiation therapy, immunotherapy, and newer treatment options like targeted therapies. The earlier the cancer is detected, the higher the probability of successful treatment, which drives the demand for surgical and therapeutic interventions.
As survival rates improve due to earlier detection and better treatment options, more women are living longer with cervical cancer, requiring ongoing treatment or surveillance. This increases the need for maintenance therapies, follow-up treatments, and new targeted therapies or immunotherapies. The market for post-treatment care is also expanding as survivorship rates increase, leading to more demand for palliative care options.
Side effects associated with cervical cancer treatments are hampering the market growth
The side effects associated with cervical cancer treatments significantly hamper market growth by impacting patient adherence to treatment regimens, reducing the overall effectiveness of therapies, and affecting the quality of life of patients. These side effects, particularly with chemotherapy, radiation therapy, and even immunotherapy, create challenges in patient management, healthcare costs, and market acceptance.
For instance, Cisplatin, a commonly used chemotherapy drug for cervical cancer, can cause severe side effects like nausea and kidney toxicity. These side effects can be so debilitating that some patients choose to skip doses or abandon the treatment altogether. This impacts the overall success of treatment and reduces market potential as patients may seek alternatives or become disillusioned with conventional therapies.
Additionally, radiation therapy can damage the ovaries, leading to early menopause and infertility. Women diagnosed at a younger age may be especially reluctant to undergo radiation due to concerns over their fertility, limiting the market demand for this treatment, especially among younger patients.
For more details on this report – Request for Sample
Cervical Cancer Treatment Market, Epidemiology Analysis
The incidence of cervical cancer is rising. DataM intelligence estimates that nearly 0.69 million incidence cases will occur worldwide in 2024. The region with the highest prevalence is the Asia-Pacific, accounting for 417.47 thousand cases. Cervical cancer is a global health issue that disproportionately affects women, particularly in low- and middle-income countries.
Cervical cancer primarily affects women in the middle-aged group, typically between the ages of 35 to 55 years. However, cervical cancer can occur at any age after puberty, and the age distribution shows some important trends. The highest incidence occurs between the ages of 45 and 49 years, with a significant number of cases also being diagnosed in women between 30 and 44 years.
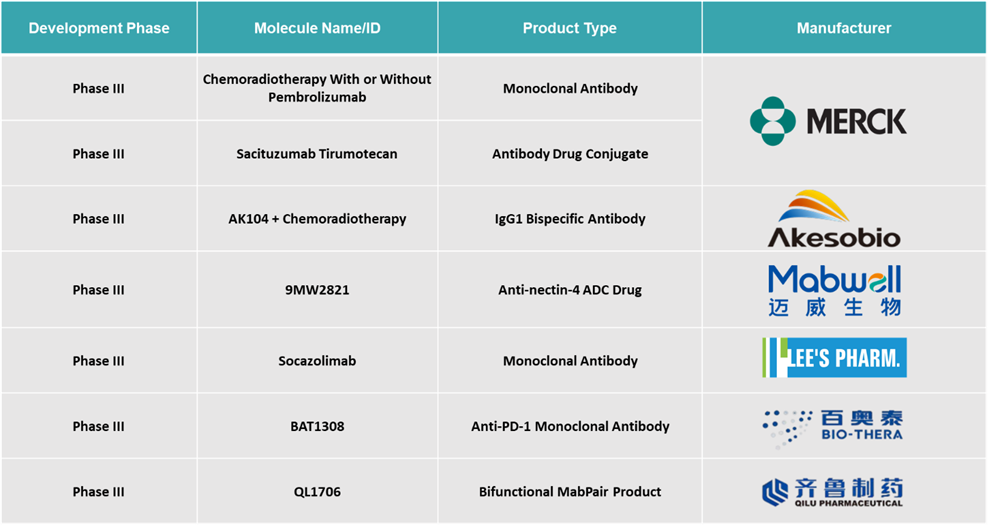
Cervical Cancer Treatment Market Pipeline Analysis
Top phase III pipeline products for cervical cancer:
Cervical Cancer Treatment Market, Segment Analysis
The global cervical cancer treatment market is segmented based on cancer type, treatment type, and region.
Treatment Type:
Chemotherapy in the treatment type segment holds the highest market share of 38.4% in 2024
Chemotherapy is often the first-line treatment for advanced-stage cervical cancer or metastatic disease. It plays a critical role when cervical cancer is diagnosed at a later stage, and surgical options (like hysterectomy) or radiation are no longer feasible or effective. In such cases, chemotherapy regimens are used to shrink tumors, control disease progression, and manage symptoms.
For instance, the most common chemotherapy regimen used for advanced cervical cancer is the combination of cisplatin (a platinum-based drug) with paclitaxel (a taxane drug). This combination has proven to be highly effective for patients with locally advanced or metastatic cervical cancer and remains the standard of care in many healthcare settings.
Chemoradiation (combining chemotherapy with radiation therapy) is the standard treatment for locally advanced cervical cancer, where the cancer is confined to the cervix and nearby tissues but has spread beyond the primary tumor site. The combination of chemotherapy and radiation works synergistically to enhance the effectiveness of treatment. For instance, the combination of cisplatin and radiation therapy has become the standard of care for patients with locally advanced cervical cancer. This combination improves overall survival rates and has been associated with a significant reduction in recurrence rates.
Cervical Cancer Treatment Market, Geographical Analysis
North America in the global cervical cancer treatment market holds the highest share of 41.5% in 2024.
North America, particularly the United States and Canada, boasts one of the most advanced healthcare systems in the world, with state-of-the-art cancer treatment centers and specialized oncology care. This infrastructure allows for the early detection, diagnosis, and treatment of cervical cancer, which is critical in improving patient outcomes.
North America has been at the forefront of implementing HPV vaccination programs, which play a key role in preventing cervical cancer. Widespread vaccination campaigns and routine screening programs have contributed to lower cervical cancer incidence rates over time. The U.S. has one of the highest vaccination rates for HPV vaccines like Gardasil. Vaccination programs, which target adolescent girls (and now boys as well), have played a key role in reducing the prevalence of HPV infections, the primary cause of cervical cancer.
Asia Pacific region in the global cervical cancer treatment market holds a share of 21.7% in 2024
Cervical cancer rates have been steadily increasing in many parts of the Asia-Pacific region, although Japan has a relatively lower incidence compared to other countries like India and China. However, the incidence is rising due to lifestyle changes and increased awareness. For instance, according to the World Health Organization, in 2024, there is an expected 417.47 thousand incidence cases in the Asia-Pacific region.
Japan boasts some of the world’s best healthcare infrastructure, with state-of-the-art cancer treatment centers and a highly skilled medical workforce. This is particularly important for advanced-stage cervical cancer treatment, where cutting-edge technologies are essential for early detection and effective treatment options like chemotherapy, radiation therapy, and immunotherapy.
Japan has strong public health education campaigns aimed at increasing awareness about cervical cancer, its risk factors (mainly HPV infection), and the importance of early detection. This, in turn, drives screening rates and leads to more early-stage diagnoses and better treatment outcomes. Awareness campaigns by organizations such as the Japanese Society of Obstetrics and Gynecology have helped inform the public about the importance of Pap tests, HPV vaccines, and regular screenings.
Cervical Cancer Treatment Market Competitive Landscape
Top companies in the cervical cancer treatment market include Amneal Pharmaceuticals LLC, Novartis AG, Merck & Co., Inc., Amgen Inc., Pfizer Inc., and among others. And the emerging market players in the market includes Mabwell (Shanghai) Bioscience Co., Ltd., Akeso Biopharma Co., Ltd., Lee's Pharmaceutical Holdings Limited., Qilu Pharmaceutical Co., Ltd., Bio-Thera Pharmaceuticals Co., Ltd. and Others.
Cervical Cancer Treatment Market Scope
| Metrics | Details | |
| CAGR | 5.4% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Cancer Type | Squamous Cell Carcinoma and Adenocarcinoma |
| Treatment Type | Chemotherapy, Immunotherapy, Radiation Therapy, Targeted Therapy, and Surgery | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials, product pipelines, and forecasts upcoming advancements in medical devices and pharmaceuticals.
- Product Performance & Market Positioning: Analyze product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: Covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyzes competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient product delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global cervical cancer treatment market report delivers a detailed analysis with 39 key tables, more than 38 visually impactful figures, and 168 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2024
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.