In the vast landscape of rare diseases, few are as complex and heartbreaking as Niemann-Pick Disease Type C (NPC). NPC is a progressive and ultimately fatal disorder that traps families in a waiting game hoping for a cure while watching symptoms unfold.
What Is Niemann-Pick Type C ?
Niemann-Pick Disease Type C (NPC) is a rare, inherited neurodegenerative disorder that affects the body's ability to metabolize cholesterol and other lipids properly within cells. When these lipids build up, especially in the brain, liver, and spleen, they cause cellular dysfunction and death, leading to a cascade of debilitating symptoms.
The disease is caused by mutations in the NPC1 or NPC2 genes, which are involved in lipid transport within cells. It follows an autosomal recessive inheritance pattern, meaning a child must inherit two copies of the faulty gene (one from each parent) to develop the condition.
From “No Treatment” to Multiple Options
For years, NPC was managed only through supportive care and off-label use of miglustat, with no FDA-approved therapies in the U.S. Patients and families had few choices, and clinicians often faced frustration due to limited tools to address a devastating disease.
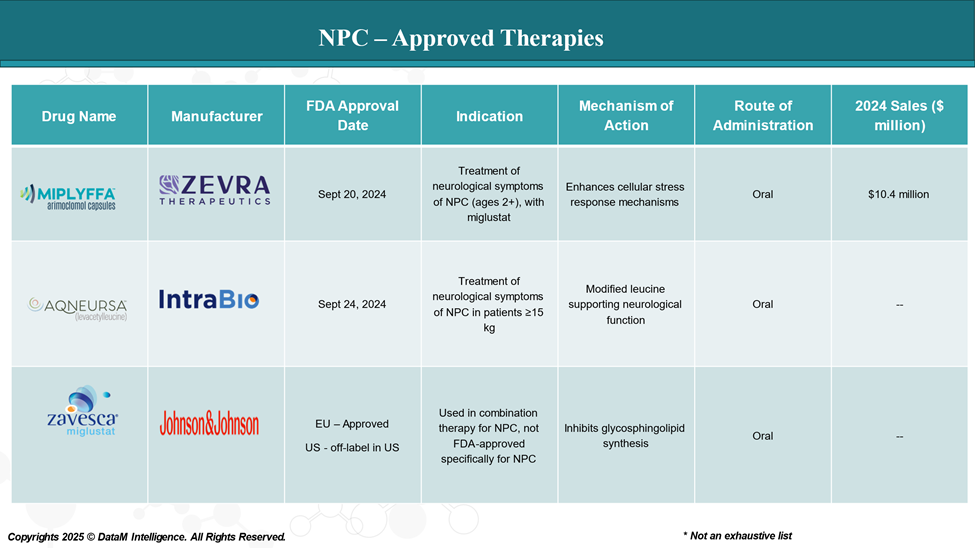
That changed dramatically in September 2024, when Miplyffa and Aqneursa both gained regulatory approval. Their arrival:
- Ended a long-standing treatment drought
- Marked the first time patients had FDA-backed therapies tailored specifically for NPC
- Encouraged global regulatory interest in rare disease therapeutics
This transition signaled a shift from a palliative approach to an active disease-management paradigm.
- Miplyffa™ (arimoclomol) - Zevra Therapeutics
Approved by the FDA for use in combination with miglustat, It helps stabilize proteins inside cells and improve lysosomal function. It works by enhancing the production of heat shock proteins, which can reduce the buildup of toxic materials.
- Aqneursa™ (levacetylleucine) - IntraBio
Aqneursa offers an alternative route by supporting neurological function through a modified amino acid formulation. It's designed to improve coordination and slow motor decline, a crucial factor for quality of life in NPC patients.
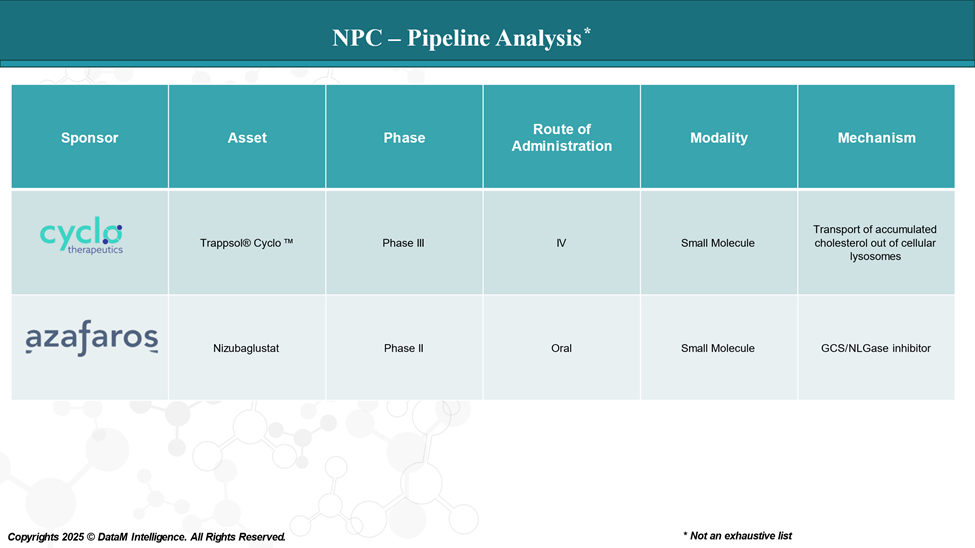
The Next Wave: Promising Therapies on the Horizon
Two emerging therapies are attracting serious attention:
- Trappsol® Cyclo™ (Cyclo Therapeutics) – Phase III
This investigational IV therapy helps remove excess cholesterol from cells. It's being studied for its ability to address the root cause of NPC, not just symptoms. Early trials are promising, and ongoing Phase III trials may offer more clarity.
- Nizubaglustat (Azafaros B. V.) – Phase II
Still in development, this oral drug aims to reduce glycosphingolipid accumulation, offering another mechanism to halt or slow disease progression. If successful, it could provide a more targeted and accessible treatment.
Why NPC Matters: Beyond the Rare
Although NPC affects only about 1 in 100,000-130,000 live births. Understanding lipid metabolism, neurodegeneration, and genetic dysfunction helps inform research into Alzheimer’s, Parkinson’s, and other neurological diseases.
Looking Ahead: From Managing to Curing
The recent drug approvals have transformed Niemann-Pick Type C from a condition with limited treatment options into a dynamic field marked by scientific innovation and therapeutic competition.
This new dynamic brings:
- Better outcomes
- Increased hope
- And most importantly, a pipeline that’s just beginning to gain steam
The next generation of therapies, whether it's substrate reduction, cholesterol mobilization, or even gene therapy, now has a clear benchmark to beat.