For decades, Duchenne Muscular Dystrophy (DMD) has been one of the most intractable inherited disorders. Characterized by progressive muscle degeneration, the disease robs children, mostly boys, of their ability to walk, breathe independently, and live into full adulthood. The root cause lies in mutations in the DMD gene, which eliminate the production of dystrophin, a protein crucial to muscle cell stability.
Until recently, therapeutic efforts were limited to symptom management: corticosteroids to delay decline, physical therapy to preserve mobility, and ventilatory support in later stages. Today, however, a new approach, gene therapy, is fundamentally challenging that status quo.
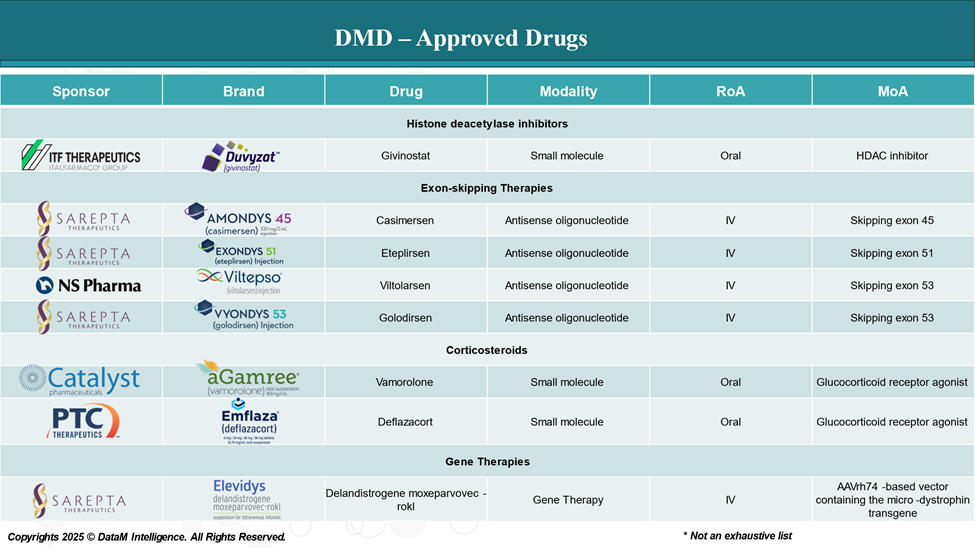
A Scientific and Strategic Shift
Gene therapy for DMD isn't about treating symptoms it's about addressing the underlying genetic defect itself. At the center of this approach is the delivery of a micro-dystrophin gene, a shortened but functional version of the massive dystrophin gene, using adeno-associated virus (AAV) vectors.
Unlike traditional therapies, gene therapy offers the possibility of a single infusion that can restore dystrophin production across skeletal and cardiac muscle. While it does not reverse existing muscle damage, early data suggest it may preserve remaining muscle tissue and slow or halt progression, especially when administered early in the disease course.
The opportunity is massive—not just from a therapeutic standpoint but from a market, regulatory, and scientific perspective. But it comes with high technical complexity, clinical risks, and long-term unknowns.
First-to-Market: Sarepta’s Elevidys
In mid-2023, Sarepta Therapeutics, in collaboration with Roche, received FDA accelerated approval for Elevidys, the world’s first gene therapy for DMD. Targeted at ambulatory boys aged 4 to 5, Elevidys delivers a micro-dystrophin gene using an AAVrh74 vector and a muscle-specific promoter to optimize tissue targeting.
This approval represents more than regulatory progress—it provides clinical validation for gene therapy in DMD. Though full approval will depend on confirmatory outcomes in ongoing Phase 3 studies, Elevidys has catalyzed momentum across the sector.
Who’s Next? The Competitive Landscape
Several companies are developing next-generation gene therapies with different constructs, vectors, and targeting strategies:
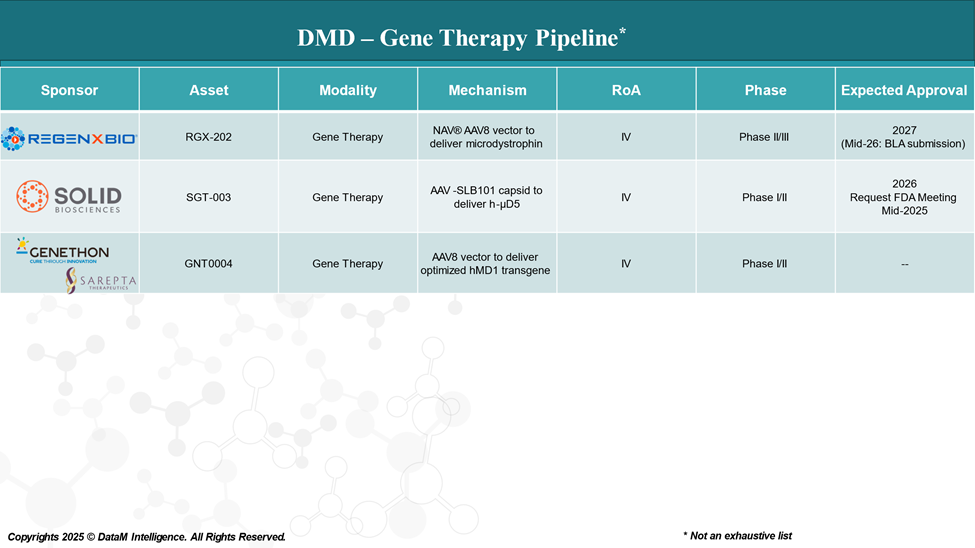
Each is exploring different strategies to improve efficacy, durability, and safety key challenges still facing the field.
Not a Cure Yet
Gene therapy has injected long-overdue momentum into DMD treatment, but it’s not without challenges.
Immune response remains a critical barrier—many patients already have neutralizing antibodies to the viral vectors used. Durability is another open question: Will a single dose last for life, or will re-dosing be necessary (and feasible)? Additionally, the therapies currently on the market and in trials do not address dystrophin’s role in the brain, where its absence may contribute to cognitive and behavioral symptoms.
Even with these limitations, gene therapy marks a significant step forward. For many families, the conversation is shifting from how to manage decline to how to plan for a future that was never promised.
Challenges That Remain
Gene therapy for DMD is a technical and biological feat—but it’s far from complete. Key challenges include:
- Immune response: Many patients already carry antibodies against AAV vectors, limiting eligibility.
- Durability of effect: It's still unclear whether a single infusion offers lifelong benefit—or whether gene expression wanes over time.
- Scalability: Manufacturing gene therapies at commercial scale, particularly for global populations, remains a major hurdle.
- CNS implications: Dystrophin is also expressed in the brain. Current therapies do not cross the blood-brain barrier, leaving cognitive symptoms unaddressed.
Next-Gen Opportunities: Beyond AAV
While AAV-based delivery has dominated early gene therapy efforts, new technologies are emerging:
- Non-viral delivery systems that bypass immune barriers
- Dual-vector strategies to deliver larger genes
- CRISPR-based gene editing, which may allow permanent correction of dystrophin mutations
- Tissue-specific promoters that minimize off-target effects
These innovations promise to expand treatment eligibility, enhance safety, and ultimately bring gene therapy to a broader population of patients—including those in later disease stages or with more complex mutations.
A Defining Moment for Rare Disease Medicine
DMD remains one of the most complex and urgent challenges in genetic medicine. But what was once a bleak outlook is now a field in motion. Gene therapy isn’t a final solution—not yet—but it’s a turning point. And for patients, families, and researchers alike, that shift offers something that’s been in short supply for far too long.