The European pharmaceutical industry is facing a growing productivity gap, which has contributed to a decline in market share for high-value, innovative drug modalities. Over the past decade, approximately 30% of essential medicines have disappeared from the EU market, with many more at risk of vanishing. This decline can be attributed to a variety of factors, including a lack of digital infrastructure for producing sensitive drug modalities, rising cost pressures, and persistent labor shortages. These challenges have significantly impacted the competitiveness of European pharma manufacturers, forcing them to adapt or risk falling behind global competitors.
Digital transformation offers a promising solution to address these challenges and bridge the productivity gap. By adopting next-generation technologies, such as real-time monitoring, AI-driven scheduling, and predictive maintenance, European pharma companies can scale up production, improve operational efficiency, and reduce costs. This eBook provides practical guidance for manufacturers looking to implement these digital strategies, supported by case studies that demonstrate the benefits of digital transformation. With these insights, EU pharma businesses can enhance their ability to compete on the global stage, particularly against lower-cost producers in Asia, and secure a sustainable future for essential medicines.
The European MedTech and digital health markets are experiencing rapid growth, with the digital health sector alone projected to reach €53.59 billion by 2029. This expansion is driven by the increasing adoption of digital treatments and care solutions, alongside supportive regulatory frameworks. While the sector faces stricter regulations, these regulations also offer a clear path for growth and innovation, creating significant opportunities for companies operating in this space. As digital health technologies, including AI, continue to evolve, the need for robust cybersecurity measures becomes more critical. Companies that focus on advanced cyber risk management and offer tailored solutions to protect healthcare technologies from both internal and external threats are well-positioned to meet the growing demand for secure healthcare solutions.

In addition to this, countries like Germany, the UK, France, and Belgium are paving the way with clear regulatory pathways and reimbursement schemes that make digital health applications more accessible to a broader range of patients and markets. This creates a fertile environment for investment opportunities and the development of innovative solutions in primary and preventative healthcare. Furthermore, the FemTech sector is gaining momentum, particularly in areas like women’s health and personalized medicine. With a projected $1 trillion economic opportunity by 2040, FemTech innovations in fields such as cardiovascular care, immunology, and personalized treatments for women present an exciting, untapped market with immense growth potential.
Digital transformation is revolutionizing industries worldwide, and the pharmaceutical sector is no exception. Although historically conservative due to strict regulatory demands, the pharmaceutical industry is increasingly recognizing the need to embrace digital technologies to remain competitive. In particular, the COVID-19 pandemic accelerated digital adoption, with pharmaceutical companies significantly investing in multichannel engagement for their sales forces. However, L.E.K. Consulting’s recent work highlights that the true potential of digital transformation extends beyond these customer-facing initiatives. A 20% or more EBITDA uplift can be achieved by optimizing areas such as production planning, inventory management, operational efficiency, and customer engagement.
While digitalization in the pharmaceutical industry remains in its early stages for many companies, there is growing confidence that more biopharma firms will implement advanced digital strategies in the near future. Data insights, AI applications, and tools like robotic process automation and intelligent automation are all part of this evolving landscape. However, a large portion of the industry still lacks a cohesive digital strategy. The article emphasizes the importance of focusing on key areas of the value chain and highlights the significant investments being made in drug discovery, clinical development, and downstream processes. European pharma companies, while lagging behind their counterparts in Asia-Pacific, are making strides in digital transformation, particularly in clinical development and drug discovery. The journey toward full digital integration, however, will require overcoming several challenges, including creating a strong strategy, securing management buy-in, and establishing effective metrics to measure success. With careful planning and execution, digital transformation can unlock substantial returns for pharmaceutical companies.
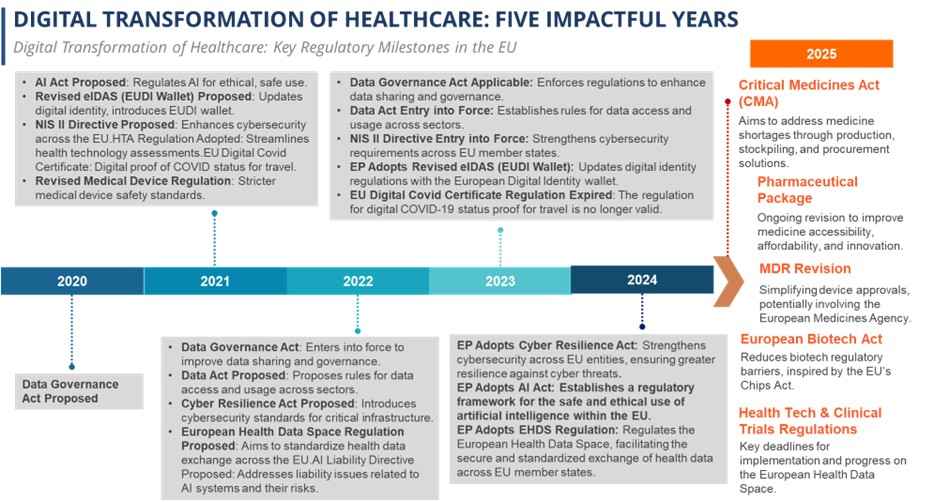
The digital transformation of healthcare in the EU is marked by a series of key regulatory milestones that are shaping the sector’s future. The proposed AI Act aims to ensure the ethical and safe use of artificial intelligence, while the Revised eIDAS (EUDI Wallet) introduces an updated digital identity system for secure access to services. The NIS II Directive enhances cybersecurity across EU member states, addressing growing concerns about digital threats. Additionally, the HTA Regulation streamlines health technology assessments, improving the efficiency of healthcare innovations. The EU Digital Covid Certificate, which facilitated travel during the pandemic, has now expired, while the Revised Medical Device Regulation enforces stricter safety standards for medical devices. With the entry into force of the Data Governance Act and Data Act, data sharing and access rules across sectors are becoming more robust. Further regulations like the Cyber Resilience Act and European Health Data Space Regulation strengthen cybersecurity for critical infrastructure and standardize health data exchange, respectively, signaling a comprehensive regulatory framework that supports the digital evolution of healthcare.