Primary Biliary Cholangitis (PBC) is a rare but serious autoimmune disease that affects the liver, mostly in middle-aged women. It used to be poorly understood and had no treatment, but in recent years, it has become a significant focus of medical research and drug development.
As more people around the world learn about PBC and new treatments become available, the search for better and safer ways to treat the disease is gaining momentum.
What is PBC?
Primary biliary cholangitis (PBC) previously known as primary biliary cirrhosis is a chronic autoimmune disease that progressively damages the small bile ducts within the liver. This leads to periportal inflammation and impaired bile flow (cholestasis). Over time, persistent cholestasis can result in liver cirrhosis and the development of portal hypertension
Despite its rarity, PBC has profound implications for patients' quality of life, making the need for innovative treatments urgent and globally significant.
The Competitive Landscape
The Race to Develop New Treatments
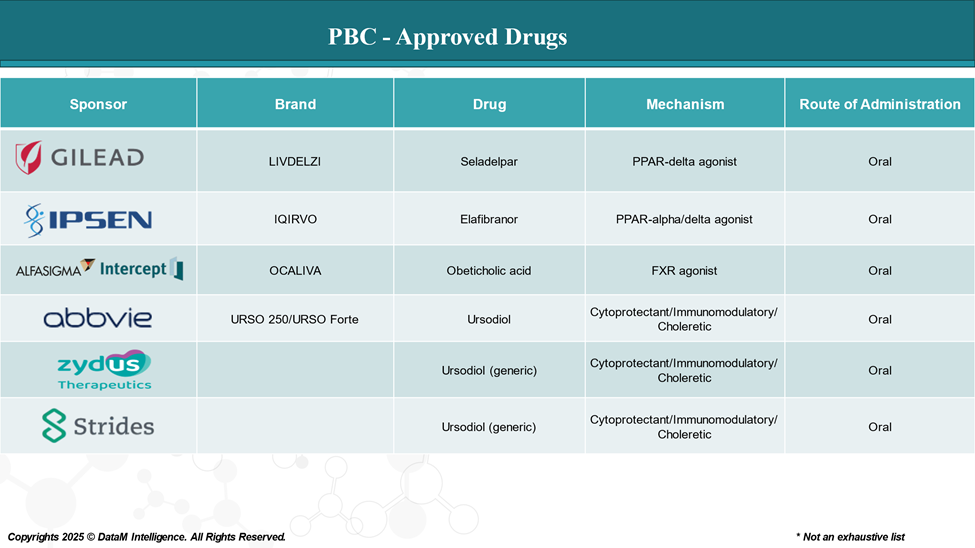
For years, ursodeoxycholic acid (UDCA) was the only FDA-approved therapy for PBC. While it remains the frontline treatment, not all patients respond well. The need for alternatives triggered a surge of innovation.
- Obeticholic Acid (Ocaliva) by Intercept Pharmaceuticals was a game-changer, gaining FDA approval in 2016 for patients with an inadequate response to UDCA.
- Gilead, Ipsen, and Intercept have entered the race with candidates targeting fibrosis pathways, bile acid regulation, and inflammation.
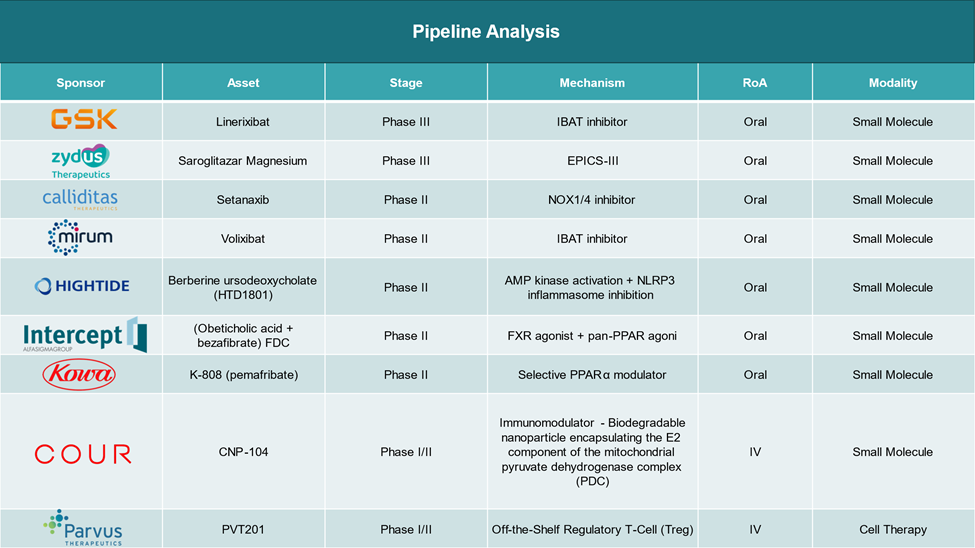
Emerging approaches include:
- PPAR agonists
- FXR agonists
- Immunomodulators
- Cell Therapy (still in early clinical stages)
Innovation Challenges & Research Competitions
Institutions and biotech accelerators are starting to host research competitions and innovation challenges to spur advancements:
- The Liver X Prize concept (currently theoretical) proposes cash incentives for breakthrough diagnostics and therapeutics in autoimmune liver disease.
- The European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) offer competitive grants and awards for PBC-focused research.
- Hackathons and bioinnovation challenges are also being designed around rare liver diseases to attract data scientists and AI researchers into the field.
Key Areas of Competition
- Biomarker Discovery: Reliable, non-invasive markers for early detection and progression
- Patient Stratification Tools: AI and precision medicine are being deployed to identify responders vs. non-responders to current therapies.
- Combination Therapy: Developing synergistic treatments using existing drugs in new ways.
- Global Trials: Multinational studies are becoming the norm, with pharma companies racing to enroll diverse populations.
The Global Impact
While the U.S. and Europe are leading in terms of research funding, countries like Japan, China, and South Korea are rapidly advancing in the PBC space, contributing to the growing global competition. Meanwhile, patient advocacy groups are playing a powerful role in pushing for transparency, access, and faster development cycles.