Multiple myeloma is a type of blood cancer originating in plasma cells white blood cells responsible for producing antibodies. In this disease, abnormal plasma cells multiply uncontrollably and secrete a single type of immunoglobulin, which can accumulate and damage surrounding bone tissue. This often leads to symptoms such as bone pain, fractures, kidney dysfunction, elevated calcium levels, anemia, and frequent infections.
Treatment Evolution: From Chemotherapy to Immunotherapy
Historically, treatment for MM was limited to chemotherapy, stem cell transplants, and supportive care. Over the past two decades, however, we've witnessed a revolution in therapy development. Current treatment strategies are often multi-drug regimens built around several core drug classes:
Backbone Therapies
- Proteasome Inhibitors:
- Bortezomib (Velcade®) – Takeda
- Carfilzomib (Kyprolis®) – Amgen
- Ixazomib (Ninlaro®) – Takeda
- Immunomodulatory Drugs (IMiDs):
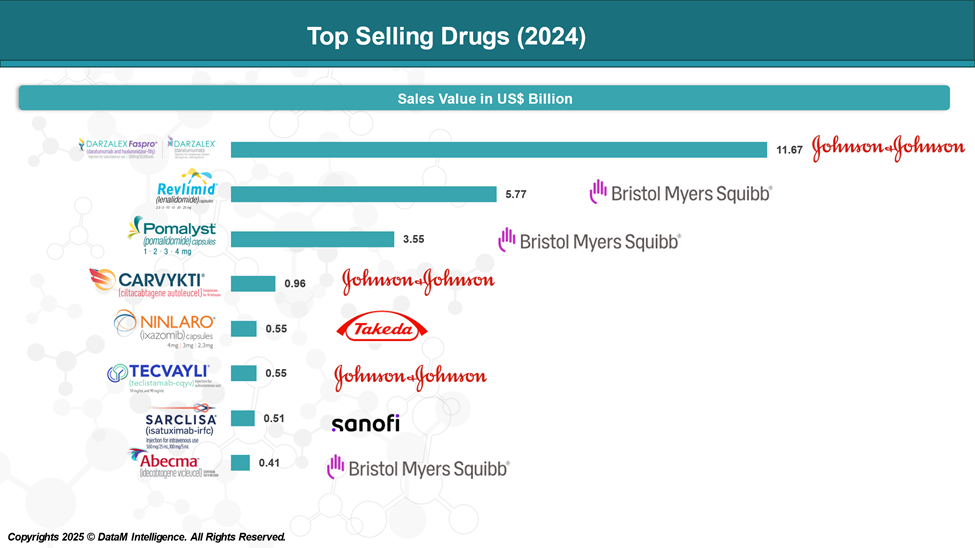
- Lenalidomide (Revlimid®) – Bristol Myers Squibb
- Pomalidomide (Pomalyst®) – Bristol Myers Squibb
- Monoclonal Antibodies:
- Daratumumab (Darzalex®) – Janssen/Johnson & Johnson
- Isatuximab (Sarclisa®) – Sanofi
These agents form the basis for multi-drug regimens used in newly diagnosed and relapsed/refractory MM patients.
Next-Generation Innovations
With resistance and relapse being common in MM, the industry is shifting focus toward next-gen therapies that go beyond traditional drug classes. Here’s what’s emerging:
CAR-T Cell Therapies
- Abecma® (idecabtagene vicleucel) – Bristol Myers Squibb & bluebird bio
First FDA-approved CAR-T therapy for MM, targeting BCMA. - Carvykti® (ciltacabtagene autoleucel) – Johnson & Johnson & Legend Biotech
Shows high response rates; a key player in late-stage treatment.
While highly effective, CAR-T therapies face logistical challenges (manufacturing time, hospital infrastructure) that limit widespread early-line use — for now.
Bispecific Antibodies
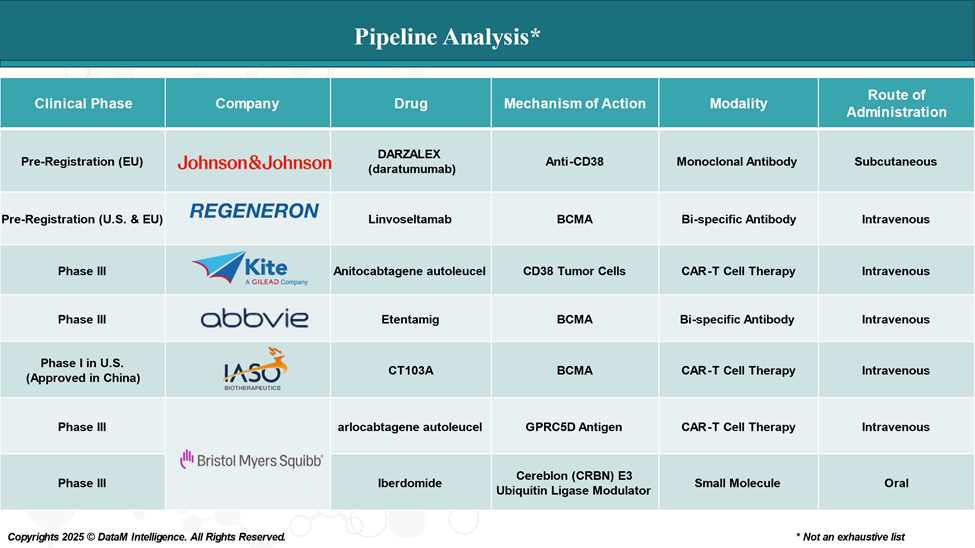
- Elrexfio™ (elranatamab) – Pfizer
- Tecvayli™ (teclistamab) – Johnson & Johnson
- TALVEY (Talquetamab) – Johnson & Johnson
These drugs engage both the cancer cell and a patient's own T-cells to drive a targeted immune response — a promising off-the-shelf alternative to CAR-T.
Antibody-Drug Conjugates (ADCs)
- Blenrep® (belantamab mafodotin) – GSK
Despite a setback with the FDA, ADCs remain an area of interest for their targeted approach and potential synergy with other therapies.
Target Trends: Beyond BCMA
While BCMA (B-cell maturation antigen) remains the dominant target, new targets are emerging:
- GPRC5D: A novel target explored by talquetamab and other bispecifics.
- FcRH5: Cevostamab, a first-of-its-kind FcRH5xCD3 T-cell engaging bispecific antibody under investigation by Roche in early-stage.
These next-gen targets aim to offer alternatives for patients who have relapsed after BCMA-directed therapies an increasingly common scenario.
Key Players and Strategic Moves
Here are some of the most influential companies shaping the MM landscape:
Bristol Myers Squibb (BMS)
- Deep MM portfolio, including Revlimid, Pomalyst, and Abecma
- Strong R&D investment in immunotherapies and combination regimens
Johnson & Johnson (Janssen)
- Dominant with Darzalex and strong in bispecifics and CAR-T via Carvykti
- Aggressively moving pipeline assets into earlier lines of therapy
Pfizer
- Gaining traction with elranatamab (Elrexfio)
- Actively expanding its hematology pipeline post-CAR-T success in ALL
Sanofi, Amgen,
- Sanofi: Sarclisa shows steady growth
- Amgen: Kyprolis continues to play a role, but innovation is needed
What’s Next?
The battle for earlier-line positioning is heating up, especially as regulators and payers look for survival and quality-of-life improvements in a cost-conscious market. The future of MM therapy will likely be defined by:
- Combination regimens using novel agents (CAR-T + bispecific or ADCs)
- Early intervention with personalized approaches
- Data-driven sequencing to overcome resistance and extend remission
Final Thoughts
Multiple Myeloma remains incurable, but it’s no longer untreatable. The influx of innovative drugs and the diversification of therapeutic mechanisms give patients more hope — and companies more strategic decisions to make.
In this competitive yet promising field, the success of companies will depend on their ability to combine innovation, market access, and operational agility. The winners will be those who develop superior therapies and ensure they are delivered to the appropriate patients at the optimal time, achieving sustained clinical outcomes.